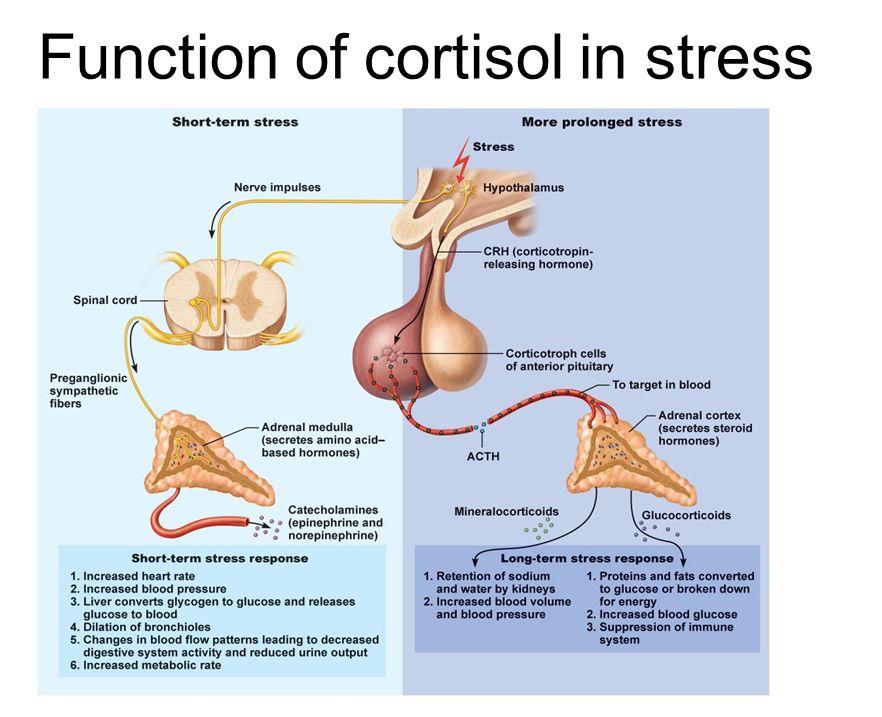
Cortisol is, in many ways, a perplexing hormone. A certain amount of cortisol is necessary for optimal health, but too much or too little can be unhealthy. During acute episodes of stress, more cortisol is released to help the body cope with physical or psychological stressors (Tomlinson 2004). Its primary functions in the body are:
-Regulation of blood glucose levels in the liver;
-Regulation of the immune system;
-Regulation of carbohydrate, protein and lipid metabolism.
Essentially, cortisol is regarded as an anti-inflammatory hormone, a blood glucose modulator, an immune-modifier, and an adaptation hormone (Chrousos 2000). Depending on diet, exercise, stress, and time of day, serum levels of cortisol can vary.
During healthy conditions, cortisol levels peak in the early morning hours (usually around 8AM) and dip to their lowest between midnight and 4AM. The complex process of cortisol biosynthesis and release is sensitive to disruption by both internal and external factors (Beishuizen 2001; Tomlinson 2004; Weerth 2003). In the face of chronic psychological stress, for example, the adrenal glands excrete an abnormal amount of cortisol in an abnormal rhythm.
Cortisol, being a catabolic hormone (a hormone that breaks down tissues), when out of balance and unregulated, can have detrimental effects on body composition. Moreover, too much cortisol can suppress the immune system, while too little can lead to autoimmunity and rheumatologic disorders (Chrousos 2000; Wu 2008; Muneer 2011; Sapolsky 2002; Tak 2011).
Cortisol receptors are expressed throughout the body, including in the brain; therefore, derangement of the biosynthesis, metabolism and release of cortisol can disrupt many physiologic systems (Beishuizen 2001).
Next week we will explore Cortisol and its relationship to weight.